Arginase-1 Antibody – 150Nd
Reactivity: Human*
Storage: Arginase-1 antibody is supplied in antibody stabilizer with 0.05% sodium azide. Store at 4°C
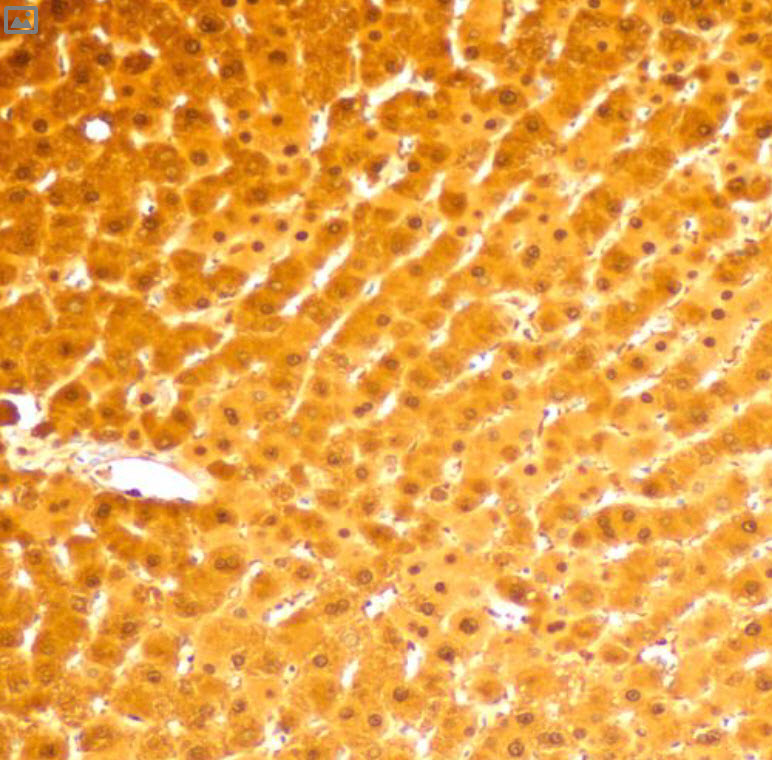
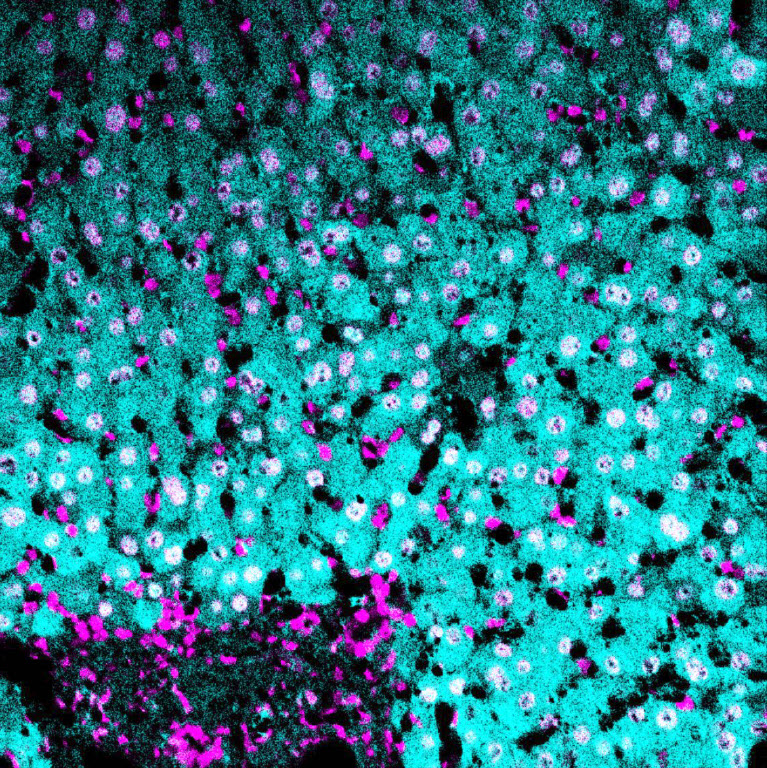
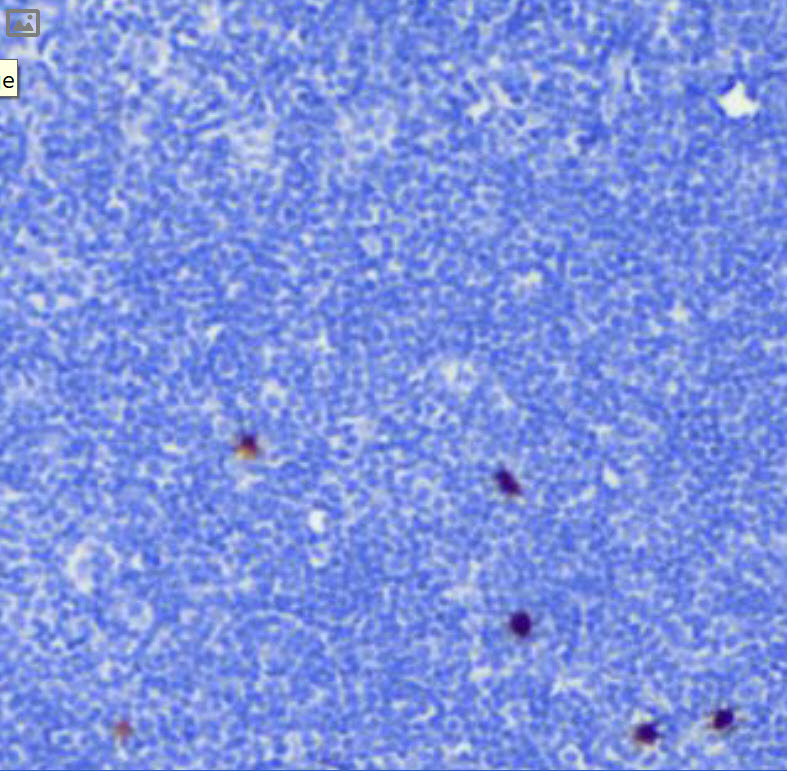
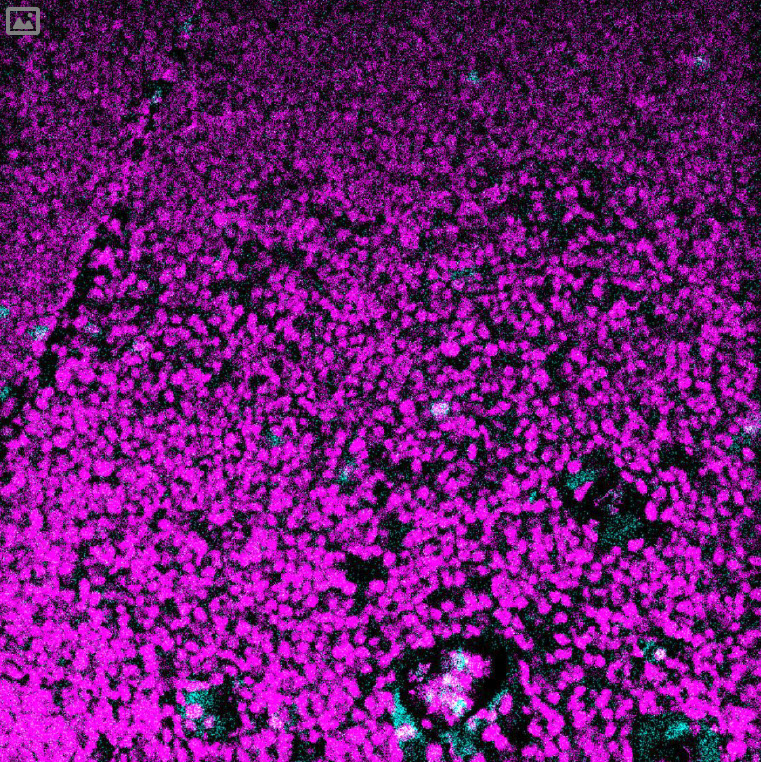
(cyan) of FFPE human thymus, counterstained with Histone H3 (magenta)
Validation: Each lot of conjugated arginase-1 antibody is quality control tested by MIBIscope™ analysis of stained tissue microarray using the appropriate positive and negative tissue field of views and are pathologist verified.
Recommended Usage: 1 uL of Arginase-1 antibody per 100 uL staining volume using the MIBI™ Staining Protocol.
For optimal results, antibody should be titrated for each desired application. Suggested starting range is 1:100.
MIBI technology: Learn more about MIBI™ Technology, a multiplex IHC technology with unmatched sensitivity and true subcellular resolution.
References
- Rodríguez, P.C., Ochoa A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008; 222:180-91.
- McGaha T.L. et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev. 2012; 249(1):135-57.
* Conjugate tested on human tissue.