Featured Publications
Scientists in the Human Tumor Atlas Network used MIBI™ spatial proteomics technology to study the progression of breast cancer, finding spatial proteomic signatures that may be predictive of which patients will develop invasive cancer.
Transition to invasive breast cancer is associated with progressive changes in the structure and composition of tumor stroma
Risom, et al., Cell 20 Jan 2022: (185), 1-12.
Key Insights
- Scientists built a spatial atlas of tumor progression in patients with ductal carcinoma in situ (DCIS) breast cancer using MIBI technology
- By analyzing dozens of proteins in nearly 80 samples, they identified a spatial proteomic signature that appears to be associated with progression to invasive breast cancer and long-term cancer relapse
- The analysis was aided by the ability to delineate cell populations and types made possible through spatial proteomics technology
Abstract
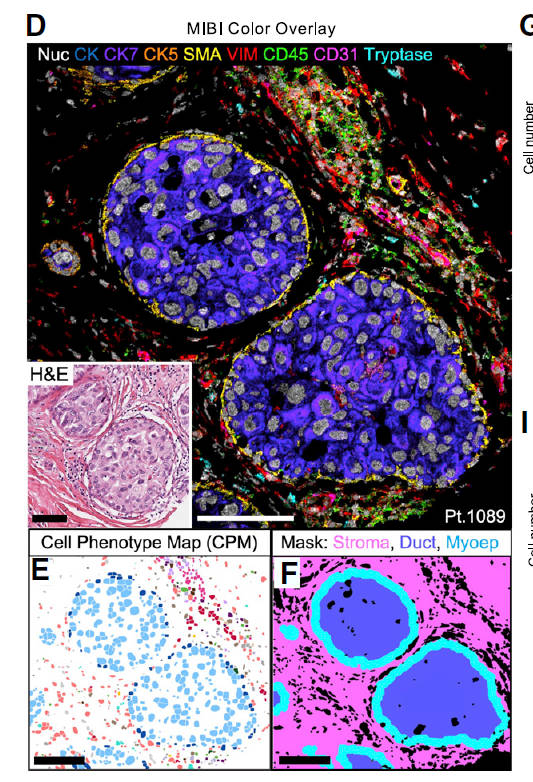
Ductal carcinoma in situ (DCIS) is a pre-invasive lesion that is thought to be a precursor to invasive breast cancer (IBC). To understand the changes in the tumor microenvironment (TME) accompanying transition to IBC, we used multiplexed ion beam imaging by time of flight (MIBI-TOF) and a 37-plex antibody staining panel to interrogate 79 clinically annotated surgical resections using machine learning tools for cell segmentation, pixel-based clustering, and object morphometrics. Comparison of normal breast with patient-matched DCIS and IBC revealed coordinated transitions between four TME states that were delineated based on the location and function of myoepithelium, fibroblasts, and immune cells. Surprisingly, myoepithelial disruption was more advanced in DCIS patients that did not develop IBC, suggesting this process could be protective against recurrence. Taken together, this HTAN Breast PreCancer Atlas study offers insight into drivers of IBC relapse and emphasizes the importance of the TME in regulating these processes.