Stanford Scientists Use MIBI Technology to Study Single-Cell Metabolic Profiles in Colorectal Carcinoma
Single-cell, metabolic regulome profiling

The challenge of studying context-dependent metabolic activity of immune cells
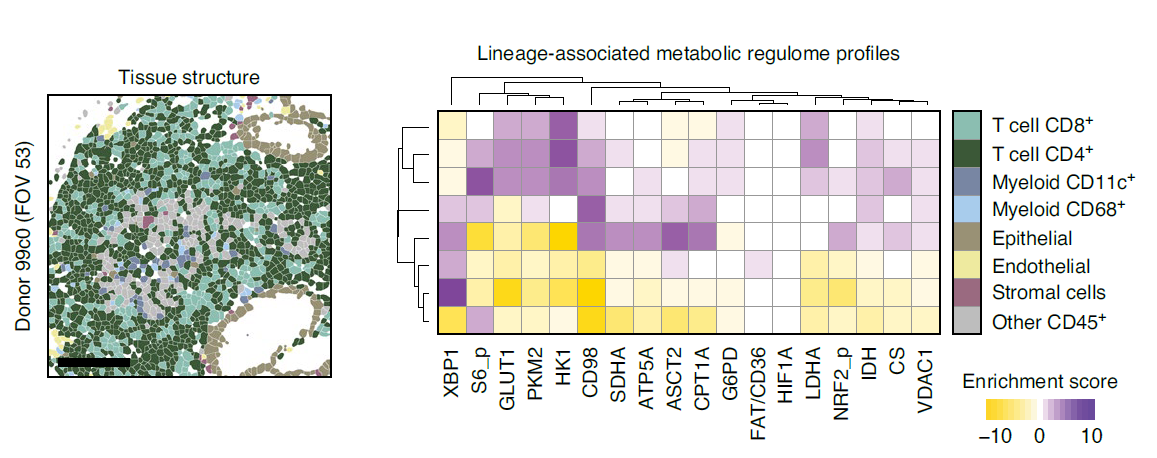
Pairing scMEP with MIBI technology enabled spatial interrogation of metabolic profiles along the tumor-immune boundary
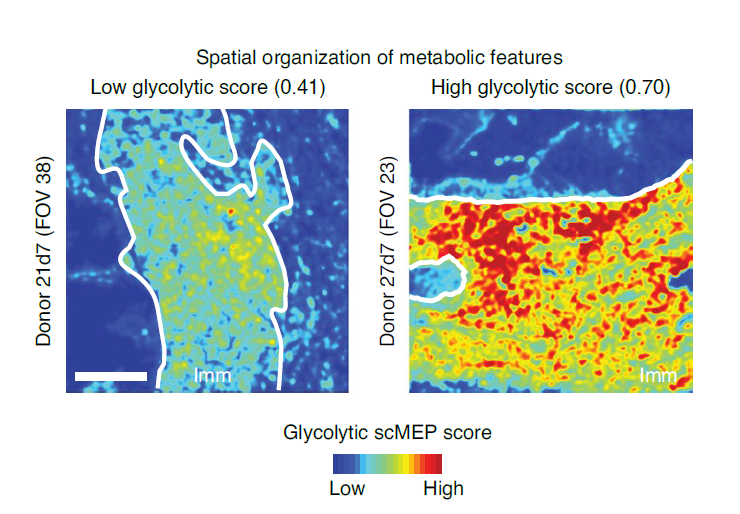
The scMEP/MIBI combination allowed the scientists to take a close look at the tumor–immune boundary, an area where the immune cell metabolism is altered by competition from the tumor. The team compared the metabolic regulatory profiles of immune cells on the tumor–immune border to those from far-away cells. They found significant evidence of “metabolic polarization of immune cells toward the tumor, which was dominated by increased expression of CD98 and ASCT2 and which could not be explained by variations in immune cell lineage,” the authors report. Interestingly, these markers have been associated with prognosis in cancer patients, and the scientists speculate that incorporating their expression with tissue features or other metabolic proteins could increase their diagnostic utility.
“These spatial analyses revealed specific exclusion of metabolic immune cell subsets from the tumor–immune boundary, demonstrating the influence of tissue architecture on metabolic regulation,” the scientists conclude. “Incorporating this new lens of single-cell metabolism into translational research promises better control of cellular alterations and dysfunction in human disease.”